Scientist Penny Vlahos explains the concept map and its development:
There are many different types of chemical contaminates, but my initial map
for educators focused on how Persistent Organic Pollutants (POP) behave and move through the atmosphere,
ocean and the biosphere. Chemicals that are classified as POPS can remain in the environment for a
significantly long time and can become stored and concentrated in plant or animal tissue (i.e., bio-accumulate).
Even though some POPs make our lives easier, they can have a negative effect on the natural environment.
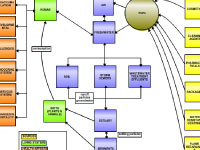
On my "To Educators" map, color-coding showed how these compounds are pulled out of global circulation either
through biodegradation or through photo-degradation by sunlight. What can happen to POPs once they are released into
the environment? If they enter the biosphere they may bio-accumulate, photo-degrade or biodegrade, or they can go into
the soil for burial. If released into the atmosphere -- where they travel much faster than in water -- POPs can be:
"dry deposited" as dust back to land or ocean, photo-degraded, precipitated as snow or rain (i.e., "wet deposited"), or
evaporate/vaporize back to the atmosphere. For example, POPs can evaporate when ambient temperatures are warm, get moved
by wind until temperatures are cooler, and then condense (a.k.a. 'global distillation effect'). If POPs are deposited in
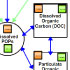
the ocean, they may be associated with dissolved organic carbon (DOC) or particulate organic carbon (POC)
in the water column, but eventually a portion of these compounds can settle into the sediments where they
remain for thousands of years.
Here's a specific example: when we use non-stick pans that are coated with Teflon, heat from cooking causes
the release of toxic compounds that can get into your food as well as float up into the atmosphere. The global
atmosphere mixes on time scales of one year, so if a compound (such as Teflon) persists in the environment
for longer than one year, it can move to Earth's poles. Indeed, we have found these compounds concentrated
in whale blubber and in tissues of animals that travel to the Arctic or Antarctic.
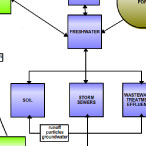
Our education team decided to refocus the map to help students and teachers understand where POPs come from
in our society (e.g. cosmetics, cleaning agents, packaging, etc.). They also felt it was important to show
how these compounds travel through the coastal environment, where most of the U.S. population lives. The
revised map also shows how humans and wildlife are affected (e.g., allergies, developmental problems, etc.)
by the consumption of POPs. Even though the revised map doesn't show the entire "life cycle" of POPs, I
think it is more accessible to middle and high students. It also builds on the coastal and urban water
cycle -- rivers, runoff, storm sewers, groundwater, estuary, etc. -- that is already taught extensively
in schools. Thus the revised map still has the important point but is now set in a familiar framework!
|