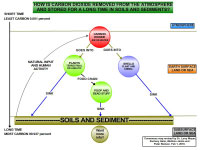
|
|
Click Images to Enlarge
|
||||||||
About this Workshop:
For this workshop, 15 graduate students from UMaine and 2 post-doctoral researchers were matched with New England-region ocean and climate scientists to improve their collective understanding of Earth's major ocean - climate systems. [more]
About this Scientist:
Lawrence Mayer is a biogeochemist - that is, he studies how organisms and materials (studied chemically) interact in earth surface environments. He and his lab use the concepts and tools of chemistry, geology and biology in these studies, placing special focus on the comparison and coupling of oceanic to terrestrial processes.
One major topic Larry studies is the bioavailability of nutritional and toxic materials (e.g., how organisms are able to glean nutrients from or be exposed to toxins) in an environmental matrix such as water or sediment. His group also studies the global carbon cycle with a focus on how the interaction between organic matter and minerals enables preservation and/or burial of organic carbon in the ocean. [more]